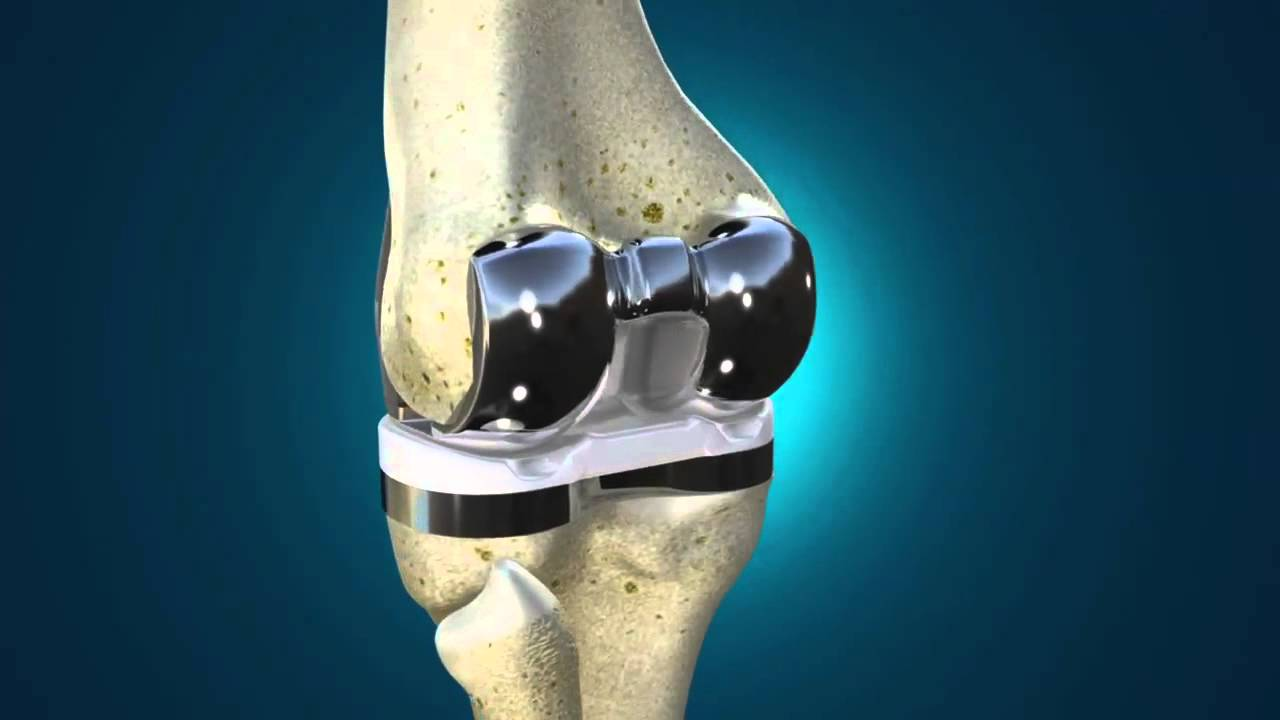
An issue with the packaging has caused a recall of artificial knee and ankle joints. Prior to implantation, the inserts must not be exposed to oxygen (for example, while being shipped and stored before your knee surgery).
Before insertion, airflow was found to have been let through the packing layers for the plastic inserts in this recall, leading to oxidation and premature wear. Although the components and inserts produced during the last eight years were vacuum-packed in oxygen-resistant bags, these bags lacked a barrier layer composed of ethylene vinyl alcohol (EVOH), significantly increasing oxygen resistance.
As a result of receiving a deeply unsettling letter from their physician, many Exactech patients have a plethora of questions. Concerned patients may wonder if they have an Exactech knee or ankle replacement and, if so, what they should do next to find out if the recall affects them.
WHY IS EXACTECH ISSUING A KNEE REPLACEMENT RECALL, AND WHAT PROBLEMS HAVE BEEN ASSOCIATED WITH IT?
When plastic components of a knee implant are exposed to oxygen before implantation, oxidation can occur, leading to premature wear and tear of the implant or even to its destruction within the patient’s body. Possible signs and symptoms include:
- An enlarged knee
- Walking pain
- The inability to put weight on your implant
- A sound like grinding or clicking
- Completely Unstable
Revision surgery may be required if the plastic insert wears out too quickly. An Exactech knee replacement lawsuit attorney would help you explore your legal options if you or a family member underwent knee surgery, including an Exactech recall implant.
EXACTECH KNEE REPLACEMENT DEVICES: IMPORTANT INFORMATION
In the first instance, EXACTECH recalls all OPTETRAK and LOGIC knee implants as well as TRULIANT knee implants manufactured since 2004.
As of 2022, all Optetrak, Optetrak Logic, and Truliant knee replacements sold by Exactech are being recalled. As a result of quality issues, nearly 150,000 knee replacements were recalled. Having had knee replacement surgery with an Exactech device within the past 18 years might mean you need to redo it.
The EXACTECH knee replacement devices were already being recalled by 2021.
All of Exactech’s knee replacement products, including the Optetrak Logic and the Truliant, were recalled in 2022 due to safety concerns. The company did, however, initiate a minor recall in 2021. As part of the initial recall, only products with at least five years remaining before expiration were affected.